Embark on a journey to master the fundamentals of chemistry with our comprehensive and simplified guide to Metals and Non-metals, Chapter 3 of CBSE Class 10 Chemistry. Designed specifically for students preparing for the CBSE 2024-25 board exams, these notes provide a clear and concise understanding of this crucial topic.
Delve into the fascinating world of elements, exploring their classification as metals, non-metals, and metalloids. Understand the unique properties of metals, including their malleability, ductility, and conductivity. Uncover the diverse characteristics of non-metals, such as their brittleness, insolubility in water, and poor electrical conductivity.
To further enhance your learning experience, we have included a downloadable PDF version of the notes, allowing you to study anytime, anywhere. Embrace simplified chemistry and propel your exam preparation to new heights with our Metals and Non-metals Class 10 notes.
| Subject | Science (Chemistry) |
| Class | 10 |
| Board | CBSE & State Boards |
| Chapter No. | 3 |
| Chapter Name | Metals and Non-metals |
| Type | Notes |
| Session | 2024-25 |
"Our greatest weakness lies in giving up. The most certain way to succeed is always to try just one more time."
- Thomas A. Edison
Metals and Non-metals Class 10 Notes
Table of Contents
Physical Properties of Metals and Non-metals
| Property | Metals | Non-metals |
| 1. Lustre | Metals have shining surfaces. | They do not have shining surfaces. • Except for Iodine. |
| 2. Hardness | They are generally hard. • Except for sodium, lithium, and potassium which are soft and can be cut with a knife. | Generally soft. • Except for Diamond, a form of carbon that is the hardest natural substance. |
| 3. State | Exist as solids. • Except for Mercury. | Exist as solids or gases. • Except for Bromine. |
| 4. Malleability | Metals can be beaten into thin sheets. • Gold and silver are the most malleable metals. | Non-metals are non-malleable. |
| 5. Ductility | Metals can be drawn into thin wires. | They are non-ductile. |
| 6. Conductor of heat and electricity | Metals are good conductors of heat and electricity. • Silver (Ag) and Copper (Cu): Best conductors of heat. • Lead (Pb), Mercury (Hg): Poor conductors of heat. | Non-metals are poor conductors of heat and electricity. • Except for Graphite. |
| 7. Density | Generally have high density and high melting points. • Except for sodium and potassium. | Have low density and low melting point. |
| 8. Sonorous | Metals produce a sound on striking a hard surface. | They are not sonorous. |
Some Exceptions:
- All metals except mercury exist as solids at room temperature.
- Metals have high melting points but gallium and caesium have very low melting points. These two metals will melt if you keep them on your palm.
- Iodine is a non-metal but it is lustrous.
- Diamond, an allotrope of carbon, is the hardest natural substance known and has a very high melting and boiling point.
- Graphite, another allotrope of carbon, is a conductor of electricity.
- Alkali metals (lithium, sodium, potassium) are so soft that they can be cut with a knife. They have low densities and low melting points.
Most non-metals produce acidic oxides when dissolved in water.
Most metals give rise to basic oxides when dissolved in water.
Chemical Properties of Metals
1. Reaction with Air (Oxygen)
Almost all metals combine with oxygen to form metal oxides.
Metal + Oxygen → Metal oxide
Examples:
- 2Cu + O2 → 2CuO
- 4Al + 3O2 → 2Al2O3
Amphoteric Oxides: Metal oxides that react with both acids, as well as bases to produce salts and water, are called amphoteric oxides. For example ZnO, Al2O3
- Al2O3 + 6HCl → 2AlCl3 + 3H2O
- Al2O3 + 2NaOH → 3NaAlO2 + H2O
Different metals show different reactivities towards oxygen.
- Metals such as potassium and sodium react so vigorously that they catch fire if kept in the open. Hence, to protect them and to prevent accidental fires, they are kept immersed in kerosene oil.
- At ordinary temperatures, the surfaces of metals such as magnesium, aluminium, zinc, lead, etc., are covered with a thin layer of oxide. The protective oxide layer prevents the metal from further oxidation.
- Iron does not burn on heating but iron filings burn vigorously when sprinkled in the flame of the burner.
- Copper does not burn, but the hot metal is coated with a black-colored layer of copper(II) oxide.
- Silver and gold do not react with oxygen even at high temperatures.
2. Reaction with Water
Metals react with water and produce a metal oxide and hydrogen gas. Metal oxides that are soluble in water dissolve in it to further form metal hydroxide. However, all metals do not react with water.
- Metal + Water → Metal oxide + Hydrogen
- Metal oxide + Water → Metal hydroxide
Metals like potassium and sodium react violently with cold water. In case of sodium and potassium, the reaction is so violent and exothermic that the evolved hydrogen immediately catches fire.
- 2K(s) + 2H2O(l) → 2KOH(aq) + H2 (g) + heat energy
- 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2 (g) + heat energy
The reaction of calcium with water is less violent. The heat evolved is not sufficient for the hydrogen to catch fire.
- Ca(s) + 2H2O(l) → Ca(OH)2 (aq) + H2 (g)
Calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of the metal.
Magnesium does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen. It also starts floating due to the bubbles of hydrogen gas sticking to its surface.
Metals like aluminium, iron, and zinc do not react either with cold or hot water. But they react with steam to form metal oxide and hydrogen.
- 2Al(s) + 3H2O(g) → Al2O3 (s) + 3H2 (g)
- 3Fe(s) + 4H2O(g) → Fe3O4 (s) + 4H2 (g)
Metals such as lead, copper, silver, and gold do not react with water at all.
3. Reaction with Acids
Metals react with acids to give salt and hydrogen gas.
Metal + Dilute acid → Salt + Hydrogen
- Hydrogen gas is not evolved when a metal reacts with nitric acid. It is because HNO3 is a strong oxidizing agent. It oxidizes the H2 produced to water and itself gets reduced to any of the nitrogen oxides (N2O, NO, NO2).
- But magnesium (Mg) and manganese (Mn) react with very dilute HNO3 to evolve H2 gas.
- Copper does not react with dilute HCl.
4. Reaction of Metals with Solutions of other Metal Salts
Reactive metals can displace less reactive metals from their compounds in solution or molten form.
Metal A + Salt solution of B → Salt solution of A + Metal B
5. Reactivity Series
The reactivity series (also called activity series) is a list of metals arranged in the order of their decreasing activities
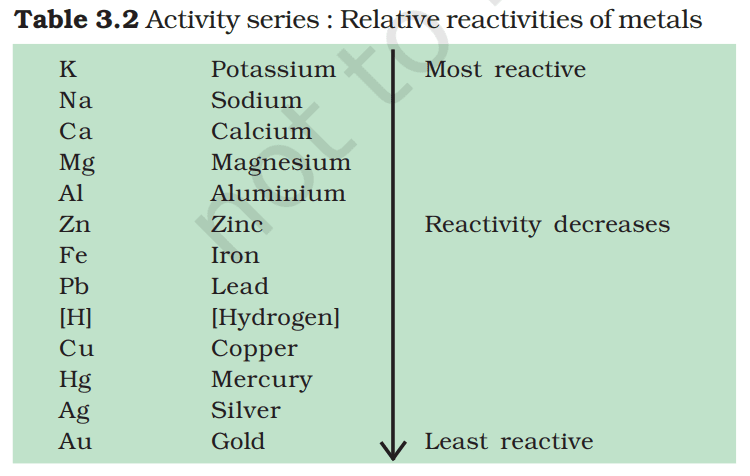
A metal placed above hydrogen in the activity series will displace hydrogen from water or acids. A metal placed at the top of the activity series would displace metal below it. Thus a more reactive metal displaces a less reactive metal from its salt solution.
Reaction of Metals and Non-metals
Formation of Sodium Chloride (NaCl)
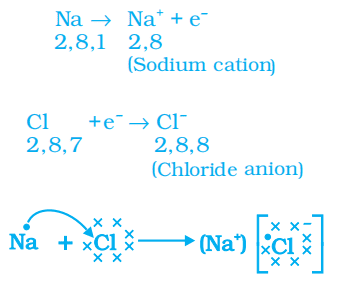
Sodium and chloride ions, being oppositely charged, attract each other and are held by strong electrostatic forces of attraction to exist as sodium chloride (NaCl).
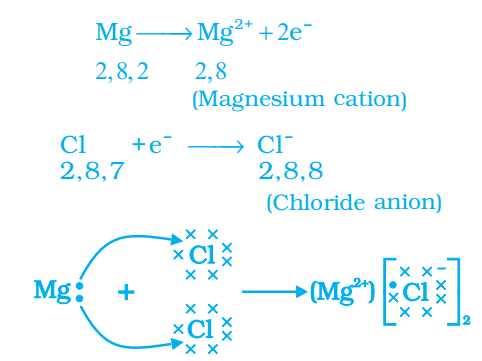
Formation of Magnesium Chloride (MgCl2)
Ionic Compounds
Ionic compounds: The compounds formed by the transfer of electrons from a metal to a non-metal are called ionic compounds or electrovalent compounds.
Properties of ionic compounds:
- Physical nature: They are solid and hard, generally brittle.
- Melting and boiling point: They have high melting and boiling point.
- Solubility: Generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
- Conduction of electricity: Ionic compounds conduct electricity in molten and solution form but not in solid state.
Occurrence of Metals
Mineral: Natural occurring chemical substances obtained by mining.
Ore: An ore is a mineral from which metal is obtained.
Gangue: Ores mined from the earth are usually contaminated with large amounts of impurities such as soil, sand, etc., called gangue.
Extraction of Metals
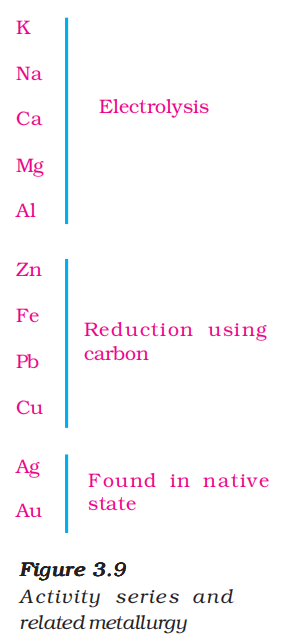
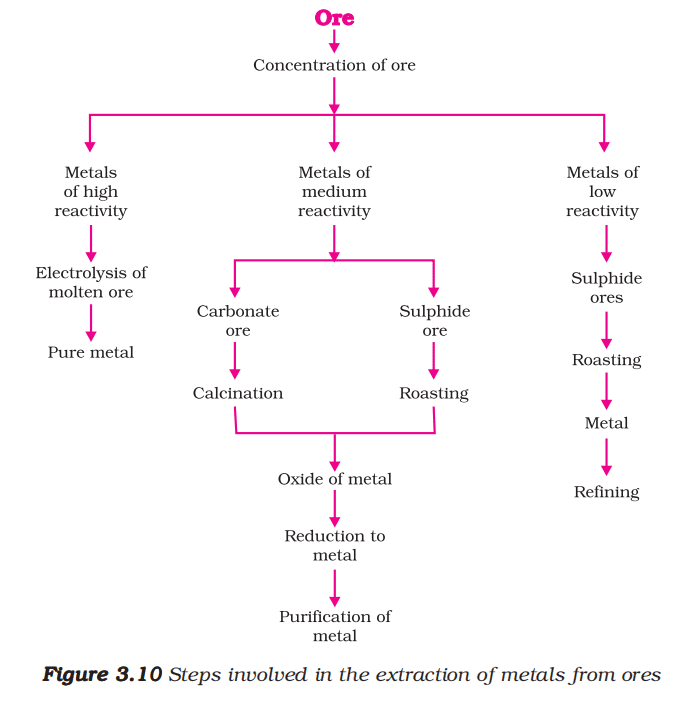
Extracting Metals Low in the Activity Series
Cinnabar (HgS) is an ore of mercury. When it is heated in air, it is first converted into mercuric oxide (HgO). Mercuric oxide is then reduced to mercury on further heating.
Roasting: 2HgS (s) + 3O2 (g) (Heat) → 2HgO (s) + 2SO2 (g)
Reduction: 2HgO (s) (Heat) → 2Hg (l) + O2 (g)
Copper which is found as Cu2S in nature can be obtained from its ore by just heating in air.
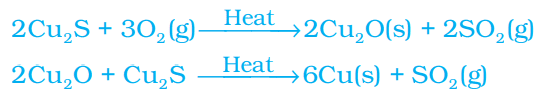
Extracting Metals in the Middle of the Activity Series
The metals in the middle of the activity series such as iron, zinc, lead, copper, are moderately reactive. These are usually present as sulphides or carbonates in nature.
| Roasting | Calcination |
| i. Ore is heated in the presence of air. | i. Ore is heated in the absence of air. |
| ii. It converts sulphide ores to oxide ores. | ii. It converts carbonate ores to oxide ores. |
| iii. For example: 2ZnS + 3O2 (heat) → 2ZnO + 2SO2 | iii. For example: ZnCO3 (heat) → ZnO + CO2 |
The metal oxides are then reduced to the corresponding metals by using suitable reducing agents such as carbon.
ZnO(s) + C(s) → Zn(s) + CO(g)
The highly reactive metals such as sodium, calcium, aluminium, etc., are used as reducing agents because they can displace metals of lower reactivity from their compounds. For example, when manganese dioxide is heated with aluminium powder, the following reaction takes place –
3MnO2(s) + 4Al(s) → 3Mn(l) + 2Al2O3(s) + Heat
These displacement reactions are highly exothermic. The amount of heat evolved is so large that the metals are produced in the molten state. In fact, the reaction of iron(III) oxide (Fe2O3) with aluminium is used to join railway tracks or cracked machine parts. This reaction is known as the thermit reaction.
Fe2O3(s) + 2Al(s) → 2Fe(l) + Al2O3(s) + Heat
Extracting Metals towards the Top of the Activity Series
For obtaining metals that are high in the reactivity series, their oxides are reduced to metals by the process of electrolysis.
For example The electrolysis of sodium chloride
At cathode: Na+ + e- → Na
At anode: 2Cl- → Cl2 + 2e-
Refining of Metals
The process of obtaining pure metal from its impure form is called the refining of metals.
Electrolytic refining of Copper:
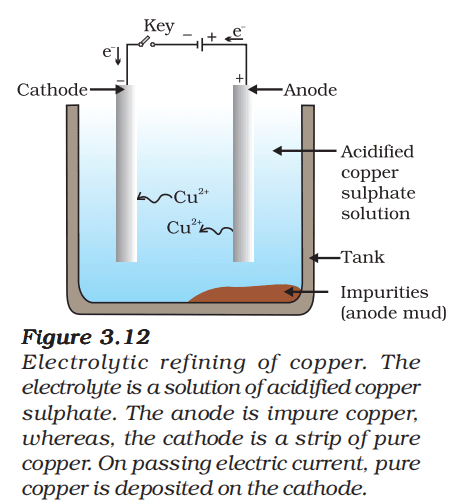
- Anode – Impure copper
- Cathode – Strip of pure copper
- Electrolyte – Solution of acidified copper sulphate
On passing the current through the electrolyte, the impure metal from the anode dissolves into the electrolyte.
Cu (Impure copper) → Cu2+ + 2e-
An equivalent amount of pure metal from the electrolyte is deposited at the cathode.
Cu 2+ + 2e- → Cu (Pure copper)
The insoluble impurities settle down at the bottom of the anode and is called anode mud.
Corrosion
- Silver articles become black after some time when exposed to air. This is because it reacts with sulphur in the air to form a coating of silver sulphide.
- Copper reacts with moist carbon dioxide in the air and slowly loses its shiny brown surface and gains a green coat. This green substance is basic copper carbonate.
- Iron when exposed to moist air for a long time acquires a coating of a brown flaky substance called rust.
- Iron articles need both air and water to rust.
Prevention of Corrosion
We can prevent the corrosion of iron by
- Painting
- Oiling and greasing
- Galvanization
- By making alloys.
Alloy: An alloy is a homogenous mixture of two or more metals or a metal and a non-metal.
Examples:
- Iron: Mixed with a small amount of carbon becomes hard and strong.
- Alloys are made to lower the melting point. Solder, an alloy of lead and tin has a lower melting point, so it is used for joining electrical wires together.
- Alloys are resistant to corrosion. Stainless steel which is made up of iron, chromium, and nickel resists corrosion. It is used for making utensils and surgical instruments.
The electrical conductivity of an alloy is less than that of pure metal.
Class 10 Science Chapter 3 Metals and Non-metals NCERT Underlined PDF
| Must Read: Metals and Non-metals Class 10 Important Questions with Answers to get an idea of the different types of questions asked from this chapter. |
| You Might Also Like: CBSE Class 10 Notes CBSE Class 10 Important Questions and Answers |
Hope you liked these notes on Class 10 Science Chapter 3 Metals and Non-metals. Please share this with your friends and do comment if you have any doubts/suggestions to share.
Thankyou author for making this wonderful notes
It’s really helpful ☺️🫶 sir!!
notes are so beautiful so elegant just looking like a wow he prabhu he hariram krishna jagganatham he kya hua aeeeee iss notes ke aage koi bol sakta he kya .com moye moye
in Amphoteric Oxides examples first one blancing is wrong tht in end it should come 3 h20
Corrected