Chemical reactions and equations are an important topic in class 10 science. Understanding the basics of chemical reactions and equations can help you understand the world around you. These simplified Chemical Reactions and Equations class 10 notes will help you grasp the concepts of chemical reactions and equations easily. For your convenience, you can also download these notes as PDF and use it for future reference.
| Subject | Science (Chemistry) |
| Class | 10 |
| Board | CBSE |
| Chapter No. | 1 |
| Chapter Name | Chemical Reactions and Equations |
| Type | Notes |
| Session | 2024-25 |
Successful people don't fear failure but understand that it's necessary to learn and grow from.
- Robert Kiyosaki
Chemical Reactions and Equations Class 10 Notes
Table of Contents
Whenever a chemical change occurs, we can say that a chemical reaction has taken place.
Activity 1.1:
- Magnesium ribbon is cleaned by rubbing it with sandpaper before burning.
- Magnesium reacts with atmospheric oxygen to develop a layer of magnesium oxide. This layer does not allow the underlying magnesium to undergo combustion.
- Magnesium ribbon burns with a dazzling white flame and changes into a white powder. This powder is magnesium oxide.
- It is formed due to the reaction between magnesium and oxygen present in the air.
- 2Mg + O2 → 2MgO
Activity 1.2:
- Lead nitrate reacts with Potassium Iodide to form Lead Iodide (yellow precipitate) and Potassium nitrate.
- Pb(NO3)2 + 2KI → PbI2 (yellow ppt)+ 2KNO3
Activity 1.3:
- Formation of hydrogen gas by the action of dilute sulphuric acid on zinc.
- Zn + 2HCl → ZnCl2 + H2
Chemical Equations
Balanced Chemical Equation:
Definition: A balanced chemical equation is an equation in which the number of atoms of various elements is equal on both sides of the equation.
Reason for balancing: An equation should be balanced due to the law of conservation of mass which states that mass can neither be created nor destroyed in a chemical reaction. That is, the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants.
Writing Symbols of Physical States:
| States | Symbol |
| gaseous | g |
| liquid | l |
| aqueous | aq |
| solid | s |
Sometimes the reaction conditions, such as temperature, pressure, catalyst, etc., for the reaction are indicated above and/or below the arrow in the equation. For example:
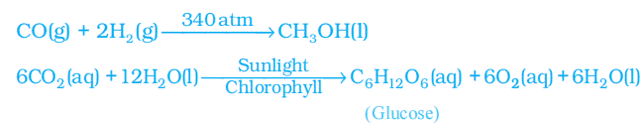
Types of Chemical Reactions
- Combination Reaction
- Decomposition Reaction
- Displacement Reaction
- Double Displacement Reaction
- Oxidation and Reduction
Some other types:
- Exothermic reaction
- Endothermic reaction
- Precipitation reaction
- Redox reaction
Combination Reaction
Definition: A reaction in which a single product is formed from two or more reactants is known as a combination reaction.
Examples:
i. Calcium oxide reacts vigorously with water to produce slaked lime (calcium hydroxide) releasing a large amount of heat.
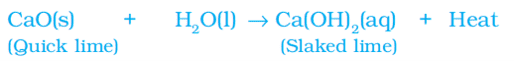
A solution of slaked lime (calcium hydroxide) is used for whitewashing walls. It reacts slowly with the carbon dioxide in the air to form a thin layer of calcium carbonate on the walls.
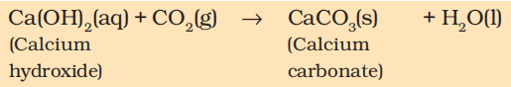
ii. Burning of coal: C + O2 → CO2
iii. Formation of water from H2(g) and O2(g): 2H2 (g) + O2 (g) → 2H2O (l)
Exothermic Reaction:
Definition: Reactions in which heat is released along with the formation of products are called exothermic chemical reactions.
Examples:
i. Burning of natural gas: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (g)
ii. Respiration: Carbohydrates are broken down to form glucose. This glucose combines with oxygen in the cells of our body and provides energy. The special name of this reaction is respiration.
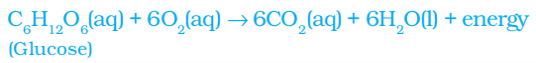
iii. The decomposition of vegetable matter into compost.
Decomposition Reaction
Definition: The reaction in which a single reactant breaks down to give simpler products is called a decomposition reaction.
Examples:
i. Ferrous sulphate crystals (FeSO4.7H2O) lose water when heated and the color of the crystals changes. It then decomposes to ferric oxide (Fe2O3), sulphur dioxide (SO2), and sulphur trioxide (SO3). Ferric oxide is a solid, while SO2 and SO3 are gases.

ii. Decomposition of calcium carbonate to calcium oxide (quick lime) and carbon dioxide on heating is an important decomposition reaction.
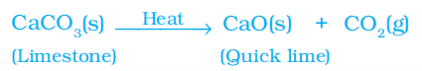
When a decomposition reaction is carried out by heating, it is called thermal decomposition.
iii. Heating of lead nitrate. You will observe the emission of brown fumes. These fumes are of nitrogen dioxide (NO2).
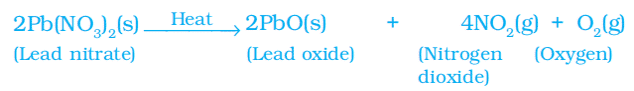
iv. Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during the electrolysis of water is 2:1.
Cathode: Hydrogen; anode: Oxygen.
Hydrogen is collected in double the amount of oxygen because water is formed by the chemical combination of hydrogen and oxygen in the ratio 2:1 by volume, so it decomposes in the same ratio.
2H2O(l) → 2H2(g) + O2(g).
v. White silver chloride turns grey in sunlight.
2AgCl (s) → 2Ag (s) + Cl2 (g)
The application of this reaction is in black-and-white photography.
Endothermic reaction:
Reactions in which energy (either in the form of heat, light, or electricity is absorbed are known as endothermic reactions.
Displacement Reaction
Definition: The reaction in which a more reactive element displaces a less reactive element from its salt solution is called displacement reaction.
Examples:
i. Iron displaces copper, from copper sulphate solution. Iron nail become brownish in color and the blue colour of the copper sulphate solution fades.
Fe + CuSO4 → FeSO4 + Cu
ii. Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
iii. Pb(s) + CuCl2(aq) → PbCl2(aq) + Cu(s)
Double Displacement Reaction
Definition: Reactions in which there is an exchange of ions between the reactants are called double displacement reactions.
Examples:
i. Na2SO4 + BaCl2 → BaSO4 (ppt) + 2NaCl
You will observe that a white substance (BaSO4), which is insoluble in water, is formed. This insoluble substance formed is known as a precipitate. Any reaction that produces a precipitate can be called a precipitation reaction.
ii. When potassium iodide solution is added to a solution of lead nitrate in a test tube, a yellow color precipitate is formed. The compound precipitated is Lead Iodide.
Pb(NO3)2 + 2KI → PbI2 + 2KNO3
Oxidation and Reduction
Definition: If a substance gains oxygen or loses hydrogen during a reaction, it is said to be oxidized. If a substance loses oxygen or gains hydrogen during a reaction, it is said to be reduced.
Examples:
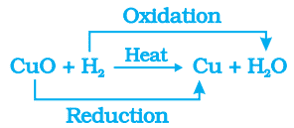
i. Oxidation of copper to copper oxide: When we heat copper powder, the surface of copper powder becomes coated with black copper(II) oxide. This is because oxygen is added to copper and copper oxide is formed.
2Cu + O2 → 2CuO
ii. Reduction of copper oxide to copper: If hydrogen gas is passed over this heated material (CuO), the black coating on the surface turns brown as the reverse reaction takes place and copper is obtained.
CuO + H2 → Cu + H2O
Redox Reactions
Definition: The reactions in which one reactant gets oxidized while the other gets reduced are called oxidation-reduction reactions or redox reactions.
Note: The substance which gets oxidized is the reducing agent and the substance which get reduced is the oxidising agent.
Examples:
i.
ii. ZnO + C → + Zn + CO
Carbon is oxidized to CO and ZnO is reduced to Zn. [Here, Carbon is the reducing agent and ZnO is the Oxidizing agent.]
iii. MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
HCl is getting oxidized to Cl2 while MnO2 is getting reduced to MnCl2. [Here, HCl is the reducing agent and MnO2 is the oxidizing agent.]
Effects of Oxidation and Reduction in everyday life
i. Corrosion:
When a metal is attacked by substances around it such as moisture, acids, etc., it is said to corrode and this process is called corrosion.
Examples:
- Rusting of iron
- The black coating on silver
- The green coating on copper
Disadvantages of Corrosion:
- Corrosion causes damage to car bodies, bridges, iron railings, ships, and to all objects made of metals, especially those of iron.
- Every year an enormous amount of money is spent to replace damaged iron.
ii. Rancidity
When fats and oils are oxidized, they become rancid, and their smell and taste change. Usually, substances that prevent oxidation (antioxidants) are added to foods containing fats and oil. Keeping food in air-tight containers helps to slow down oxidation.
Chips manufacturers usually flush bags of chips with a gas such as nitrogen to prevent the chips from getting oxidized.
| Must Read: Chemical Reactions and Equations Class 10 Important Questions & Answers to get an idea of the different types of questions asked from this chapter. |
| You Might Also Like: CBSE Class 10 Notes CBSE Class 10 Important Questions and Answers |
Hope you liked these notes on Class 10 Chemistry Chapter 1 Chemical Reactions and Equations. Please share this with your friends and do comment if you have any doubts/suggestions to share.
Sir, you might add oxidation agent and reduction agents in this …
Okay, I have added.
Sir , you writen wrong in not of oxidising agent or reducing agent
=> There is writen that reduced substance is reducing agent
Now corrected 👍
Sir issme download ka option nahi aata h kya aap hme Aisa notes provide kr skte h jo download ho ske
Ye lo https://www.printfriendly.com/p/g/fE88yH
Isme metal reactivity series add kijiye
Potassium
Sodium
Magnesium
Aluminum
Zinc
Iron
Lead
Hydrogen
Copper
Mercury
Silver
Gold
Where is pyqs
Sir, could you please add the details about the pungent smell of Sulphur oxides? Additionally, there appears to be an error in the note regarding oxidizing and reducing agents.(oxidising agent is replaced with reducing agent).
Sir please teach us class 10 science chemistry. We need your help sir . Find some time .
Sir, you could have given pdf form of the notes so that we could get its offline copy.
How to Download PDF from CBSE Guidance Website
https://youtu.be/zjocgf0LzUM