Have you been struggling to remember the important questions of Chapter 1 Chemical Reactions and Equations for Class 10 Science? If so, you've come to the right place. In this post, we will provide you with comprehensive and helpful questions to help you study better and ace your exams.

| Subject | Science (Chemistry) |
| Class | 10 |
| Board | CBSE |
| Chapter No. | 1 |
| Chapter Name | Chemical Reactions and Equations |
| Type | Important Questions & Answers |
| Session | 2024-25 |
Chemical Reactions and Equations Class 10 Important (Extra) Questions and Answers
Q. No. 1) Multiple choice questions (MCQs):
i. Which of the following reaction(s) is an endothermic reaction?
a. Burning of coal
b. Decomposition of vegetable matter into compost
c. Process of respiration
d. Decomposition of calcium carbonate to form quick lime and carbon dioxide.
e. Dilution of Sulphuric acid
Ans. Option (d).
ii. You are given the solution of lead nitrate. In order to obtain a yellow precipitate you should mix with it a solution of ______.
a. Potassium chloride
b. Potassium iodide
c. Potassium nitride
d. Potassium sulphide
Ans. Option (b).
iii. Which of the following are exothermic processes?
- Reaction of water with quick lime
- Dilution of an acid
- Evaporation of water
- Sublimation of camphor (crystals)
a. 1 and 2
b. 2 and 3
c. 1 and 4
d. 3 and 4
Ans. Option (a).
iv. Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during the electrolysis of water is
a. 1:1
b. 2:1
c. 4:1
d. 1:2
Ans. Option (b).
v. Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by the liberation of heat. This process is called slaking of lime. Which among the following is/are true about slaking of lime?
- It is an endothermic reaction.
- It is an exothermic reaction.
- The pH of the resulting solution will be more than seven.
- The pH of the resulting solution will be less than seven.
a. 1 and 2
b. 2 and 3
c. 1 and 4
d. 3 and 4
Ans. Option (b).
vi. Identify the endothermic reaction(s) among the following:
- 6CO2 + 12H2O → C6H12O6 + 6O2 + 6H2O
- Na2CO3 + 2HCl → 2NaCl + CO2 + H2O
- C6H12O6 + 6O2 → 6CO2 + 6H2O
- CaCO3 → CaO + CO2
Options
a. Only 1
b. Only 4
c. Only 2 and 3
d. Only 1 and 4
Ans. Option (d)
vii. Which of the following statements about the given reaction are correct?
3Fe + 4H2O → Fe3O4 + 4H2
- Iron metal is getting oxidized.
- Water is getting reduced.
- Water is acting as a reducing agent.
- Water is acting as an oxidizing agent.
a. 1, 2, and 3
b. 3 and 4
c. 1, 2, and 4
d. 2 and 4
Ans. Option (c).
viii. Sunita takes about 2 g ferrous sulphate crystals in a dry boiling tube and heats the boiling tube over the flame of a burner or spirit lamp as shown in the figure.
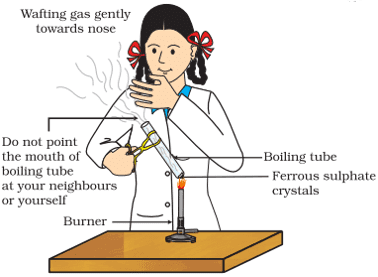
The color of crystals after heating is:
a. Black
b. Brown
c. Green
d. Orange
Ans. Option (b)
ix. The silver chloride is placed under the sunlight as shown in the figure. The color of silver chloride after some time is:
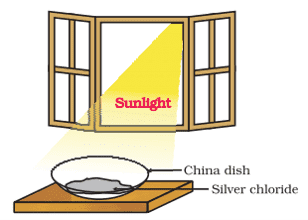
a. Black
b. Green
c. Grey
d. Yellow
Ans. Option (c).
x. A small amount of copper powder is heated as shown in the figure.
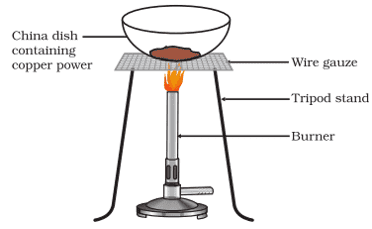
Which reaction shows the above reaction?
a. 4Cu + O2 → 2Cu2O
b. CuO + H2 → Cu + H2O
c. 2Cu + O2 → 2CuO
d. None of the above
Ans. Option (c).
xi. The following reactions are carried out in open vessels.
- 2Cu (s) + O2 (g) → 2CuO (s)
- Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
- 2FeSO4 (s) → Fe2O3 (s) + SO2 (g) + SO3 (g)
Which of the following correctly shows if the weight of the reaction vessel and contents increases, decreases, or remains the same after the reaction as compared to before the reaction?
| Options | Reaction A | Reaction B | Reaction C |
| a. | decreases | remains the same | increases |
| b. | remains the same | increases | decreases |
| c. | increases | decreases | increases |
| d. | increases | remains the same | decreases |
Ans. Option (d)
xii.
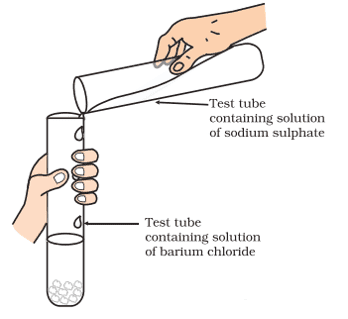
Identify the product which represents the solid state in the above reaction.
a. Barium chloride
b. Barium sulphate
c. Sodium chloride
d. Sodium sulphate
Ans. Option (b)
Q. No. 2) Assertion-Reason-Based Questions
For the following questions, two statements are given: one labeled Assertion (A) and the other labeled Reason (R).
Select the correct answer to these questions from the codes (a), (b), (c), and (d) as given below:
a. Both (A) and (R) are true and (R) is the correct explanation of the assertion.
b. Both (A) and (R) are true, but (R) is not the correct explanation of the assertion.
c. (A) is true, but (R) is false
d. (A) is false, but (R) is true.
i. Assertion (A): A white-washed wall develops a coating of calcium carbonate after a few days.
Reason (R): Calcium oxide on the wall reacts slowly with carbon dioxide in the air.
Ans. Option (c) [Calcium hydroxide reacts slowly with the carbon dioxide in the air to form a thin layer of calcium carbonate on the walls. Ca(OH)2 + CO2 → CaCO3 + H2O]
ii. Assertion (A): Following is a balanced chemical equation for the action of steam on iron:
3Fe + 4H20 → Fe3O4 + 4H2
Reason (R): The law of conservation of mass holds good for a chemical equation.
Ans. Option (a).
iii. Assertion (A): While equalizing the number of atoms to balance a chemical equation, we alter the formula of the compounds or elements involved in the reaction.
Reason (R): A chemical equation is balanced by making the number of atoms of each element equal on both sides of the arrow.
Ans. Option (d).
iv. Assertion (A): In the reaction: MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
HCl is getting oxidized while MnO2 is getting reduced.
Reason (R): The process in which oxygen is added to a substance is called oxidation whereas the process in which oxygen is removed from a substance is called reduction.
Ans. Option (a).
Q. No. 3) While Abhi was about to burn a magnesium ribbon in the chemistry laboratory, the teacher asked him to clean the ribbon with sandpaper before burning it. What could be the reason for the above instruction by the teacher? After burning the magnesium ribbon, Abhi obtained a white-colored residue. Name this residue. What type of chemical reaction has occurred? Write a balanced chemical equation to explain the reaction.
Ans. Magnesium reacts with atmospheric oxygen to develop a layer of magnesium oxide. This layer does not allow the underlying magnesium to undergo combustion.
The residue is Magnesium Oxide.
The type of chemical reaction is a Combination reaction.
2Mg + O2 → 2MgO.
Q. No. 4) Dil. HCl is added to Zn granules.” How will you prove that chemical change has taken place
here? Support your response with two arguments.
Ans. The following observations help us to determine whether a chemical reaction has taken place:
- Bubbles of gas/ Evolution of gas
- Change in color (Zn - silvery grey to black)
- Change in temperature
Q. No. 5) Define chemical reaction. State four observations that help to determine whether a chemical reaction has taken place or not. Write one example of each observation with a balanced chemical equation.
Ans. A chemical reaction is a reaction that represents a chemical change.
Four observations are:
- Change in colour: Cu + 2AgNO3 (colorless) → CuNO3 (blue)+ 2Ag
- Evolution of gas: CaCO3 (s) → CaO (s) + CO2 (g)
- Formation of precipitate: Pb(NO3)2 + 2KI → PbI2 (yellow ppt)+ 2KNO3
- Change in temperature: CaO + H2O → Ca(OH)2 + Heat
Q. No. 6) Which among the following are physical or chemical changes?
a. Evaporation of petrol
b. Burning of liquefied petroleum gas (LPG)
c. Heating of an iron rod to red hot.
d. Curdling of milk.
e. Sublimation of solid ammonium chloride.
Ans. a. Physical change (Because evaporation is a physical change).
b. Chemical change (Because burning is always a chemical change).
c. Physical change (Because this is reversible).
d. Chemical change (Because chemically milk and curd are different).
e. Physical change (Because here only change of form is occurring. No new substance is formed.)
Q. No. 7) a. Define a balanced chemical equation. Why should an equation be balanced?
b. Write a balanced chemical equation for the following reactions:
i. Phosphorus burns in the presence of chlorine to form phosphorus pentachloride.
ii. Burning of natural gas.
iii. The process of respiration.
Ans. a. Balanced chemical equation is an equation in which the number of atoms of various elements are equal on both sides of the equation.
An equation should be balanced due to the law of conservation of mass which states that mass can neither be created nor destroyed in a chemical reaction. That is, the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants
b. i. 2P + 5Cl2 → 2PCl5
ii. CH4 + 2O2 → CO2 + 2H2O
iii. C6H12O6 + 6O2 → 6CO2 + 6H2O
Q. No. 8) A clear solution of slaked lime is made by dissolving Ca(OH)2 in an excess of water. This solution is left exposed to air. The solution slowly goes milky as a faint white precipitate form. Explain why faint white precipitate forms, and support your response with the help of a chemical equation.
Ans. Calcium hydroxide reacts with Carbon dioxide present in the atmosphere to form Calcium carbonate which results in milkiness/white precipitate/ formation of Calcium carbonate.
Ca(OH)2 + CO2 → CaCO3 (white ppt) + H2O
Q. No. 9) a. A + BC → AC + B
b. AB + CD → AC + BD
Identify the types of reaction mentioned above in (a) and (b). Give one example for each type in the form of a balanced chemical equation.
Ans. a. Displacement reaction: Fe + CuSO4 → FeSO4 + Cu.
b. Double displacement reaction: Na2SO4 + BaCl2 → BaSO4 + 2NaCl.
Q. No. 10) Identify the type of chemical reaction in the following statement and define each of them:
a. Digestion of food in our body.
b. Rusting of iron.
c. Heating of manganese dioxide with aluminium powder.
d. Dilute hydrochloric acid is added to sodium hydroxide solution to form sodium chloride and water.
Ans. a. Decomposition reaction: It is a chemical reaction in which a compound is broken down into simple substances.
b. Oxidation: Reactions that involve the gain of oxygen or loss of hydrogen.
c. Displacement reactions: The reaction in which a more reactive element displaces a less reactive element from its salt solution is called displacement reaction.
d. Neutralization reaction: The reaction in which acid reacts with a base to form salt and water.
Q. No. 11) 2g of ferrous sulphate crystals are heated in a dry boiling tube.
i. List any two observations.
ii. Name the type of chemical reaction that takes place.
iii. Write the chemical equation for the reaction.
Ans. i. A reddish-brown solid is obtained, the smell of burning sulphur.
ii. Decomposition reaction.
iii. 2FeSO4 → Fe2O3 + SO2 + SO3.
Q. No. 12) On heating blue colored powder of copper nitrate in a boiling tube, copper oxide, oxygen gas, and a brown gas X are formed.
a. Write a balanced chemical equation of the reaction.
b. Identify the brown gas X evolved.
c. Identify the type of reaction.
d. What could be the pH range of the aqueous solution of the gas X?
Ans. a. 2Cu(NO3)2 → 2CuO + 4NO2 + O2
b. The brown gas is nitrogen dioxide.
c. It is a decomposition reaction.
d. NO2 is an oxide of non-metal. On dissolving in water, it will produce an acidic solution. So the pH of the solution will be less than 7.
Q. No. 13) A group of students carried out electrolysis of acidified water in the laboratory. By the end of the experiment, two gases were collected in the test tubes at both electrodes.
a. Name the gases collected at the cathode and the anode respectively.
b. The gas collected in one test tube is double the volume of gas collected in the other. Name this gas. Give reason for your answer.
c. Write a balanced chemical equation to represent the electrolysis of water.
Ans. a. Cathode: Hydrogen; anode: Oxygen.
b. The gas is hydrogen. This is because water is formed by the chemical combination of hydrogen and oxygen in the ratio 2:1 by volume, so it decomposes in the same ratio.
c. 2H2O(l) → 2H2(g) + O2(g).
Q. No. 14) Why do we store silver chloride in dark-colored bottles? What happens when silver chloride decomposes in presence of sunlight? Write the chemical reaction involved. Identify the type of reaction. What is the application of this reaction?
Ans. We store silver chloride in dark-colored bottles because it undergoes photochemical decomposition reaction in presence of sunlight.
2AgCl (s) → 2Ag (s) + Cl2 (g)
It is a decomposition reaction.
The application of this reaction is in black-and-white photography.
Q. No. 15) Write one example for each decomposition reaction carried out with the help of:
i. Electricity
ii. Heat
iii. Light
Ans. i. 2H2O(l) → 2H2 (g) + O2 (g).
ii. CaCO3 (s) → CaO (s) + CO2 (g)
iii. 2AgBr (s) → 2Ag (s) + Br2 (g)
Q. No. 16) When potassium iodide solution is added to a solution of lead nitrate in a test tube, a precipitate is formed.
i. What is the color of this precipitate? Name the compound precipitated.
ii. Write the balanced chemical equation for this reaction.
iii. List any two types of reaction in which this reaction can be placed.
Ans. i. Yellow color precipitate is formed. The compound precipitated is Lead Iodide.
ii. Pb(NO3)2 + 2KI → PbI2 + 2KNO3
iii. Double decomposition and precipitation reaction.
Q. No. 17) Write the balanced chemical equations for the following reactions:
a. Sodium carbonate on reaction with hydrochloric acid in equal molar concentrations gives sodium chloride and sodium hydrogen carbonate.
b. Sodium hydrogen carbonate on reaction with hydrochloric acid gives sodium chloride, and water and liberates carbon dioxide.
c. Copper sulphate on treatment with potassium iodide precipitates cuprous iodide (Cu2I2), liberates iodine gas, and also forms potassium sulphate.
d. Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
e. A piece of sodium metal is added to absolute ethanol to form sodium ethoxide and hydrogen gas.
f. Iron (III) oxide on heating with carbon monoxide gas reacts to form solid iron and liberates carbon dioxide gas.
g. Hydrogen sulphide gas reacts with oxygen gas to form solid sulphur and liquid water.
h. Thermit reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
i. Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride.
j. Chlorine gas is passed in an aqueous potassium iodide solution to form potassium chloride solution and solid iodine.
k. Ethanol is burnt in air to form carbon dioxide, and water, and releases heat.
l. Nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773 K to form ammonia gas.
m. Sodium hydroxide solution is treated with acetic acid to form sodium acetate and water.
n. Ethanol is warmed with ethanoic acid to form ethyl acetate in the presence of concentrated sulphuric acid.
o. Ethene is burnt in the presence of oxygen to form carbon dioxide, and water and releases heat and light.
Ans. a. Na2CO3 + HCl → NaCl + NaHCO3
b. NaHCO3 + HCl → NaCl + H2O + CO2
c. 2CuSO4 + 4KI → Cu2I2 + 2K2SO4 + I2
d. Pb(CH3COO)2 + 2HCl → PbCl2 + CH3COOH
e. 2Na + 2C2H5OH → 2C2H5ONa + H2
f. Fe2O3 + 3CO → 2Fe + 3CO2
g. 2H2S + O2 → 2S + 2H2O
h. Fe2O3 + 2Al → Al2O3 + 2Fe + Heat
i. 3Mg + N2 → Mg3N2
j. 2KI + Cl2 → 2KCl + I2
k. C2H5OH + 3O2 → 2CO2 + 3H2O + Heat
l. N2 + 3H2 → 2NH3
m. NaOH + CH3COOH → CH3COONa + H2O
n. C2H5OH + CH3COOH → CH3COOC2H5 + H2O
o. C2H4 + 3O2 → 2CO2 + 2H2O + Heat + Light
Q. No. 18) Identify the oxidizing agent (oxidant) in the following reactions:
a. Pb3O4 + 8HCl → 3PbCl2 + Cl2 + 4H2O
b. Mg + 2H2O → Mg(OH)2 + H2
c. CuSO4 + Zn → Cu + ZnSO4
d. V2O5 + 5Ca → 2V + 5CaO
Ans. (a) Pb3O4
(b) H2O
(c) CuSO4
(d) V2O5
Q. No. 19) Identify the reducing agent in the following reactions:
a. 4NH3 + 5O2 → 4NO + 6H2O
b. H2O + F2 → HF + HOF
c. Fe2O3 + 3CO → 2Fe + 3CO2
d. 2H2 + O2 → 2H2O
Ans. a. NH3
b. Water (H2O) as F2 is getting reduced to HF
c. CO
d. H2
| You Might Also Like: CBSE Class 10 Notes CBSE Class 10 Important Questions and Answers |
I hope these questions were helpful to you in preparing Class 10 Science Chapter 1: Chemical Reactions and Equations for your exams. Please share this with your friends and do comment your thoughts/doubts/suggestions... in the comment section below.
In question 9 (a) and (c) option is same . Or why?
Thank you for noticing and telling me. I have changed option (a) now.
But sir isn’t it supposed to be black??
Sir how do I download this pdf
In question 1 vii) Shouldn’t the answer be white? As far as i know the color of ferrous sulphate crystals after dehydration changes from light green to white.
No. The color of Ferrous Sulphate Crystals is green. After heating, it decomposed to Ferric oxide which is Brown in color.
Actually, ferrous sulphate crystals first turn white when they lose their water molecules. On further heating, it changes to brown.
If i do all these question i would able to attend all other questions that came in board examination?
In question vi why iii opstion is not a endothermic reaction 🤔
In question vi why iii opstion is not a endothermic reaction 🤔
Because c is respiration. In respiration energy is released, so it is exothermic.
If i do all these question i would able to attend all other questions that came in board examination?
Yes!
Sir mein aap ka lecture ka line kiya hua aur important question answer samajh aur yaad karun toh kya 95 % ajaayega kya
If i do all these question i would able to attend all other questions that came in board examination?please reply
On question 17 balance the equation
Question f Is wrong can u check the balancing of it
It’s correct. Fe 2, O 6, C 3
U just changed it I see
Sir,
My question is, can I study from oswaal 11th question banks to practice for state board?
SUPPPPPPPER SIR, Aapka website bestest hai , cbse sample paper 2023-2024 wale me aapke chemical reaction se jitne question aaye hai wo sab hai isme already. Thanks for making this website
Sir if ferrous sulphate crystals which possess green colour are heated, the formation of white powder of ferrous sulphate takes place. So why they will convert directly into brown coloured ferric oxide?
You’re right, the statement that heating green ferrous sulphate crystals directly results in brown ferric oxide (Fe2O3) is inaccurate.
When you heat ferrous sulphate crystals (FeSO4·7H2O), they lose their water molecules. This results in white ferrous sulphate (FeSO4).
If you continue heating the white ferrous sulphate further, it decomposes into ferric oxide (Fe2O3), sulphur dioxide (SO2), and sulphur trioxide (SO3):
2FeSO4 -> Fe2O3 + SO2 + SO3
Ferric oxide is responsible for the reddish-brown color observed at this stage.
Sir apne burning of natural gas tah equation Asa deya haii CH4 + O2 → CO2 + 2H2O
But CH4 + 2O2 give CO2 + 2H20
which one is right ✅
Thik kar diye ab
It’s useful for my SSE science board exam 2024
CH4+2o2 gives CO2+2H2O
Sir lead nitrate ke decompositions me jo nitrogen dioxide hota haj vi brown fumes nikalti hai kya copper nitrate ke decompositions me bhi aisa hi hai sir pls reply
Isme ta pdf download ka option nahi hain
How to Download PDF from CBSE Guidance Website
https://youtu.be/zjocgf0LzUM