The CBSE Class 10 Science Exam 2025 was conducted today, 20th February 2025, from 10:30 AM to 1:30 PM. Students across India appeared for the exam, and now, the most awaited CBSE Class 10 Science Answer Key 2025 with detailed solutions is here! This post provides set-wise answers to help students analyze their performance and estimate their scores.
CBSE Class 10 Science Answer Key 2025 – Set 1
Our expert teachers have solved the CBSE Class 10 Science Board Exam 2025 and provided a detailed answer key based on the NCERT syllabus and CBSE marking scheme.
📌 Exam Date: 20th February 2025
📌 Exam Time: 10:30 AM – 1:30 PM
📌 Subject: Science
📌 Set Numbers: Set 1
📌 Series: 3EGFH, Q.P. Code: 31/3/1
📌 Official CBSE Website: www.cbse.gov.in
📌 Unofficial Answer Key PDF: [Download Here] (Link will be updated soon)
CBSE Class 10 Science 2025 Answer Key (All Sections)
The Science exam consisted of three sections:
- Physics – Numericals and conceptual questions.
- Chemistry – Chemical equations, reactions, and theory-based questions.
- Biology – Diagram-based and application-based questions.
Below are the detailed answers for all sections:
SECTION A
Select and write the most appropriate option out of the four options given for each of the questions no. 1 to 20. There is no negative marking for incorrect response. (20 × 1 = 20)
1. Consider the following chemical equation:
pAl + qH₂O → rAl₂O₃ + sH₂
To balance this chemical equation, the values of ‘p’, ‘q’, ‘r’, and ‘s’ must be respectively:
(A) 3, 2, 2, 1
(B) 2, 3, 3, 1
(C) 2, 3, 1, 3
(D) 3, 1, 2, 2
Ans. Option (C)
2. Which of the given options represents a family of salts?
(A) NaCl, Na₂SO₄, CaSO₄
(B) K₂SO₄, Na₂SO₄, CaSO₄
(C) NaNO₃, CaCO₃, Na₂CO₃
(D) MgSO₄, CuSO₄, MgCl₂
Ans. Option (B)
3. The most common method of extraction of metals from their oxide ores is:
(A) Reduction with carbon
(B) Reduction with hydrogen
(C) Reduction with aluminium
(D) Electrolytic reduction
Ans. Option (A)
4. Given below are the structures of some hydrocarbons. Select the two structures which are related to each other from the given options:
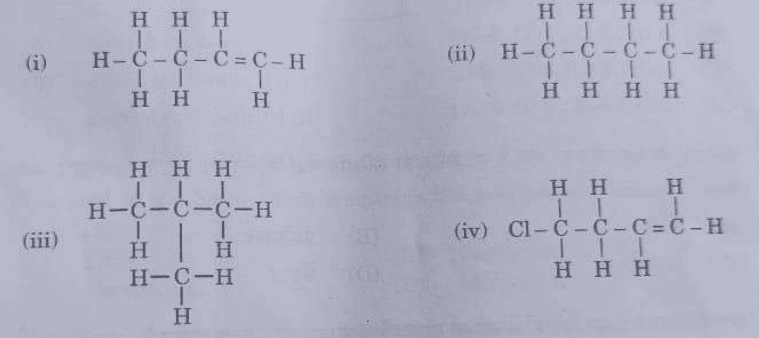
(A) (i) and (iv)
(B) (ii) and (iv)
(C) (ii) and (iii)
(D) (i) and (iii)
Ans. Option (C)
5. Choose the incorrect statement about the common reaction used in hydrogenation of vegetable oils.
(A) It is an addition reaction.
(B) It takes place in the presence of nickel or palladium catalyst.
(C) The product contains only single bonds between carbon atoms.
(D) It is an addition reaction which occurs in the presence of an acid catalyst.
Ans. Option (D)
6. Match Column-I with Column-II and select the correct option from the choices provided.
| Column-I | Column-II |
| a. Site of fertilisation of egg with the sperm | (i) Vagina |
| b. Site of implantation of embryo | (ii) Uterus |
| c. Site of entry of sperm into the female reproductive tract | (iii) Oviduct |
| d. Site through which the waste materials generated by the developing embryo are removed | (iv) Placenta |
| (v) Cervix |
(A) a-(ii), b-(i), c-(v), d-(iv)
(B) a-(iii), b-(i), c-(v), d-(iv)
(C) a-(iv), b-(ii), c-(iii), d-(i)
(D) a-(iii), b-(ii), c-(i), d-(iv)
Ans. Option (D)
7. Select a pair of bisexual flowers from the following:
(A) Papaya and mustard
(B) Hibiscus and mustard
(C) Hibiscus and papaya
(D) Hibiscus and watermelon
Ans. Option (B)
8. The part of the brain which maintains the posture and balance of the body is:
(A) Pons
(B) Cerebrum
(C) Cerebellum
(D) Medulla
Ans. Option (C)
9. The plant hormone present in greater concentration in the areas of rapidly dividing cells is:
(A) Auxin
(B) Cytokinins
(C) Gibberellins
(D) Abscisic acid
Ans. Option (B)
10. The gastric glands present in the wall of the stomach release:
(A) Mucus and Trypsin
(B) Pepsin and Trypsin
(C) Mucus and Pepsin
(D) Pepsin and Salivary amylase
Ans. Option (C)
11. Absolute refractive index of water and glass is 4/3 and 3/2 respectively. If the speed of light in glass is 2 x 10^8 m/s, the speed of light in water is:
(A) 9/4 m/s
(B) 7/3 m/s
(C) 16/9 m/s
(D) 9/8 m/s
Ans. (C) 2 x 10^8 x (4/3) / (3/2) = 2 x 10^8 x 8/9 = 16/9 x 10^8 m/s [OPTIONS Mistake] Marks will be given to all who attempted this question.
However, among the given options, the closest match is: (C) 16/9 m/s (ignoring the 10^8 factor)
12. When a beam of white light passes through a region of very fine dust particles, the colour of light that scatters the most in that region is:
(A) red
(B) orange
(C) blue
(D) yellow
Ans. Option (C)
13. A wire of length (l) is gradually stretched so that its length increases to (3l). If its original resistance is (R), then its new resistance will be:
(A) 3R
(B) 6R
(C) 9R
(D) 27R
Ans. Option (C)
14. Which one of the following statements is not true about a bar magnet?
(A) It sets itself in north-south direction when suspended freely.
(B) It has attractive power for iron filings.
(C) It produces magnetic field lines.
(D) The direction of magnetic field lines inside a bar magnet is from its north pole to its south pole.
Ans. Option (D)
15. The strength of magnetic field produced inside a long straight current carrying solenoid does not depend upon:
(A) number of turns in the solenoid
(B) direction of current flowing through the solenoid
(C) material of the core filled inside the solenoid
(D) radius of the coil of the solenoid
Ans. Option (B)
16. Other than the abiotic components, which of the given biotic components are not required to make an aquarium with small herbivorous fishes a self-sustaining system?
(i) Aquatic plants and aquatic animals
(ii) Terrestrial plants and terrestrial animals
(iii) Decomposers as bacteria and fungi
(iv) Consumers as clown fishes and sea urchins
(A) (i) and (iv)
(B) (ii) and (iii)
(C) (i) and (iii)
(D) (ii) and (iv)
Answer: (D) (ii) and (iv)
For Questions number 17 to 20, two statements are given - one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C) and (D) as given below.
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
17. Assertion (A): Hydrogen gas is not evolved when a metal reacts with nitric acid.
Reason (R): Nitric acid is a strong reducing agent and reduces the hydrogen produced in the reaction to water.
Answer: (C) Assertion (A) is true, but Reason (R) is false.
18. Assertion (A): In our actions of writing or talking, our nervous system communicates with the muscles.
Reason (R): Cranial nerves and spinal nerves form the peripheral nervous system.
Answer: (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (A).
19. Assertion (A): Magnetic field lines around a bar magnet never intersect each other.
Reason (R): Magnetic field produced by a bar magnet is a quantity that has both magnitude and direction.
Answer: (B) Both Assertion (A) and Reason (R) are true and Reason (R) is not the correct explanation of Assertion (A).
20. Assertion (A): Use of jute bags for shopping reduces pollution.
Reason (R): Jute is biodegradable and its bag may be reused as and when needed.
Answer: (A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
Section B
Questions no. 21 to 26 are Very Short Answer Type questions.
21. (a) List the possible sources of energy required in decomposition reactions. Illustrate any one with a suitable example.
OR
(b) What is observed when hydrated ferrous sulphate crystals are heated in a dry boiling tube? Give balanced chemical equation(s) of the reactions(s) that occur(s).
Ans. (a) Possible sources of energy required in decomposition reactions:
- Heat
- Light
- Electricity
Example: 2H2O → 2H2 + O2 ( electrolysis of water)
OR
(b) When hydrated ferrous sulphate crystals are heated in a dry boiling tube:
The reaction is: 2FeSO₄(s) → Fe₂O₃(s) + SO₂(g) + SO₃(g)
The crystals lose water of crystallization and turn into anhydrous ferrous sulphate and the colour of the crystals changes.
22. (a) Write the formula of the ions which (i) acids, and (ii) bases generate in water solutions.
(b) Dry HCl gas does not change the colour of dry litmus paper. Why?
Ans. (a) Formulas of ions generated in water solutions:
- Acids: H+ (hydrogen ions)
- Bases: OH- (hydroxide ions)
(b) Dry HCl gas does not change the color of dry litmus paper because:
Litmus paper changes color in the presence of hydrogen ions (H+) in aqueous solutions, not in the presence of dry HCl gas.
23. State the main function of veins in human circulatory system. Why do they not need thick walls?
Ans. Main function of veins in human circulatory system:
- To carry deoxygenated blood back to the heart.
Veins do not need thick walls because:
- They carry blood at lower pressure than arteries.
24. (a) Explain how the proteins control the 'characteristics' in an organism with the help of an example of 'tallness' trait in pea plant.
(b) Name the section of DNA that controls the 'characteristics' in an organism.
Ans. (a) Proteins control characteristics in an organism by encoding genetic information in DNA, which determines traits like tallness in pea plants.
Example: The gene for tallness in pea plants codes for a protein that promotes stem elongation.
(b) Section of DNA that controls characteristics: Gene
25. (a) A student has difficulty in reading his textbooks but can read the blackboard clearly while sitting in the last row. Name the defect of vision the student is suffering from. List two reasons due to which this defect arises. Write the nature of the lenses required to correct this defect.
Ans. (a) Defect of vision:
- Hypermetropia (far-sightedness)
Reasons:
- the focal length of the eye lens is too long, or
- the eyeball has become too small.
Nature of lenses required:
This defect can be corrected by using a convex lens of appropriate power.
OR
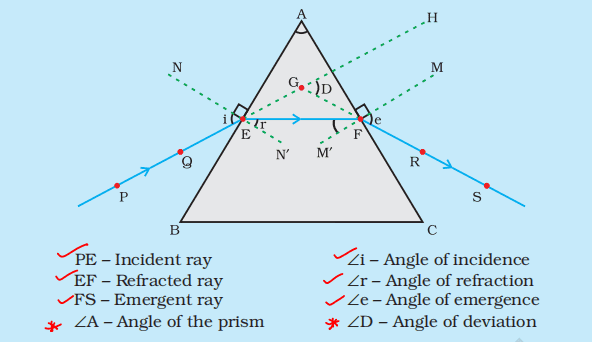
(b) Draw a ray diagram to show the path of a ray of light falling obliquely on one of the refracting faces of a triangular glass prism and mark the angle of deviation on it.
Ans.
26. An electric kettle is rated 230 V; 1000 W. Calculate the resistance of its heating element when in operation.
Ans. Power (P) = Voltage (V) x Current (I)
We are given:
P = 1000 W
V = 230 V
I = P / V
= 1000 W / 230 V
= 4.35 A
Now, we can use Ohm's Law to calculate the resistance:
Resistance (R) = Voltage (V) / Current (I)
= 230 V / 4.35 A
= 52.87 Ω
Section C
Questions no. 27 to 33 are Short Answer Type questions.
27. (a) What is a reactivity series of elements? How is it developed? Arrange the following elements as they are arranged in the reactivity series:
Aluminum, Calcium, Copper, Lead
(b) Write balanced chemical equation to show the reaction of iron (III) oxide (Fe₂O₃) with aluminium.
Ans. (a) Reactivity series of elements:
- A series of elements arranged in order of their reactivity, with the most reactive elements at the top.
- Developed by observing the reactions of elements with water, acids, and other elements.
Arrangement of elements:
- Calcium (most reactive)
- Aluminum
- Copper
- Lead (least reactive)
b. 2Al + Fe₂O₃ → Al₂O₃ + 2Fe
28. (a) Common salt is an important raw material for various chemicals of daily use. State in brief the method of preparation of (i) Sodium hydroxide, and (ii) Sodium hydrogen carbonate from common salt. Write balanced chemical equations of the reactions that occur.
OR
(b) Design an experimental set-up to demonstrate that "Alcohol and glucose contain hydrogen but are not categorized as acids". Also give the reason to justify this fact.
Ans. (a) i. When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed– chlor for chlorine and alkali for sodium hydroxide.
2NaCl (aq) + 2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
ii. It is produced using sodium chloride as one of the raw materials.
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
b. Experimental Set-up:
- 3 test tubes with litmus paper
- Add HCl, ethanol, and glucose to separate tubes
- Observe color change
Observation:
- HCl: litmus turns red
- Ethanol and glucose: litmus remains blue
Reason:
Acids (HCl) release H+ ions, turning litmus red. Ethanol and glucose do not.
29. On the basis of the characteristics of the processes given in the brackets in each case, differentiate between the following:
(a) Products of breakdown of pyruvate in aerobic and anaerobic respiration in human beings (product(s) of the processes)
(b) Respiration and photosynthesis in plants (gas released)
(c) Respiration in terrestrial animals and fishes (organs involved)
Ans. (a) Products of breakdown of pyruvate in aerobic and anaerobic respiration:
- Aerobic respiration: CO₂ and H₂O
- Anaerobic respiration: Lactic acid (in muscles) or ethanol and CO₂ (in yeast)
(b) Respiration and photosynthesis in plants:
- Respiration: CO₂ released
- Photosynthesis: O₂ released
(c) Respiration in terrestrial animals and fishes:
Fishes: gills
Terrestrial animals: lungs
30. A pure pea plant having round (R), yellow (Y) seeds is crossed with another pure pea plant having wrinkled (r), green (y) seeds. Subsequently (F_1) progeny is self-pollinated to obtain (F_2) progeny.
(a) What do the seeds of (F_1) generation look like?
(b) Give the possible combinations of traits in seeds of (F_2) generation. Also give their ratio.
(c) State the reason of obtaining seeds of new combination of traits in (F_2) generation.
Ans. (a) The F₁ generation will have all round, yellow (RrYy) seeds because the dominant traits (R for round and Y for yellow) mask the recessive traits (r for wrinkled and y for green).
(b) Possible combinations of traits in (F₂) generation and their ratio
- When the F₁ progeny (RrYy) is self-pollinated, the F₂ generation will show a 9:3:3:1 ratio, following the Mendelian dihybrid cross.
- Final Ratio: 9 (Round, Yellow) : 3 (Round, Green) : 3 (Wrinkled, Yellow) : 1 (Wrinkled, Green)
(c) Reason for obtaining seeds with new combinations in (F₂) generation
- This happens due to independent assortment of genes, as described by Mendel’s law of independent assortment.
- The alleles for seed shape (R, r) and seed color (Y, y) assort independently during gamete formation, leading to new combinations in the F₂ generation.
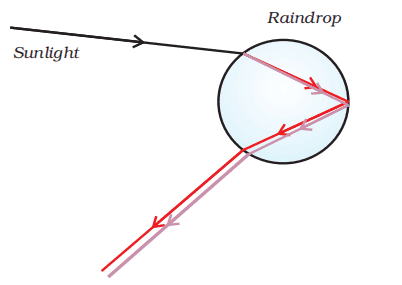
31. What is a rainbow? Draw a labelled diagram to show the formation.
Ans. A rainbow is a natural spectrum appearing in the sky after a rain shower. It is caused by the dispersion of sunlight by tiny water droplets, present in the atmosphere.
32. Consider a rectangular cardboard having two holes P and Q through which a current-carrying circular loop has been inserted as shown in the diagram.
(a) Make this diagram on your answer sheet and draw three magnetic field lines, one each passing through the points 1 (near P), 2 (at the centre of the loop) and 3 (near Q).
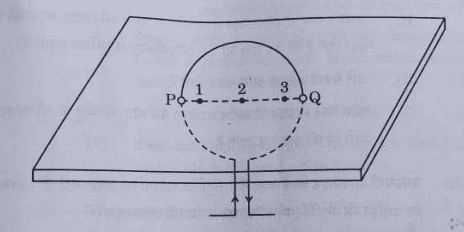
(b) List two factors on which the intensity of the magnetic field produced at the centre of the loop depends.
(c) Name the rule you will apply to determine the direction of the magnetic field produced due to a current-carrying straight conductor.
Ans. a.
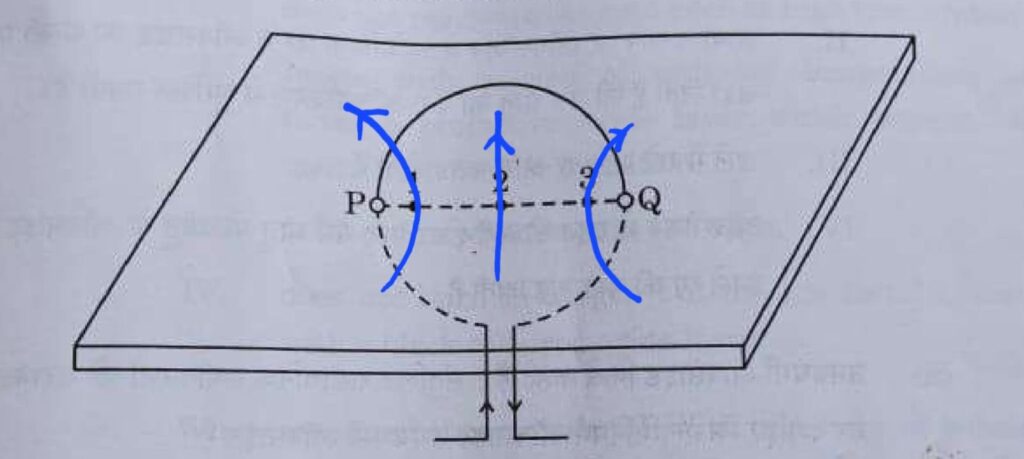
b. The intensity of the magnetic field produced at the centre of the loop depends on:
- Current: The magnitude of the current flowing through the loop.
- No. of turns: More the number of turns in the coil, more the magnitude of magnetic field produced.
c. To determine the direction of the magnetic field produced due to a current-carrying straight conductor, we apply the Right-Hand Thumb Rule.
33. (a) "In a food chain energy flow is unidirectional." Give two reasons for the given statement.
(b) If 10,000 J energy is available at the producer level, how much energy will be available to the secondary consumers? Give reason to justify your answer.
Ans. a. The flow of energy generally is Sun → Producer → Hervibore → Carnivore. Since the flow is progressively from one trophic level to another and does not revert back, it is said to be unidirectional. Further, the available energy decreases in the higher trophic levels making it impossible for energy to flow in the reverse direction.
b. Energy available at producer level = 10,000 J
Energy available at primary consumer level = 10,000 x 0.1 = 1,000 J
Energy available at secondary consumer level = 1,000 x 0.1 = 100 J
Section D
Questions no. 34 to 36 are Long Answer Type questions.
34. (a) (i) Consider the following metals:
K, Ca, Al, Cu, Ag, Fe
Select from the above metals, a metal which
I. does not react with oxygen even at high temperature.
II. reacts with oxygen at ordinary temperature and forms a protective oxide layer which prevents the metal from further oxidation.
III. catches fire when kept in the open.
IV. does not burn in oxygen but the hot metal is coated with a black coloured oxide layer.
(ii) What are amphoteric oxides? With the help of balanced chemical equations show that aluminium oxide is an amphoteric oxide.
(iii) What are alkalis? Give one example.
OR
(b) (i) With the help of balanced chemical equations state the process of extracting (I) mercury from its ore called cinnabar, and (II) copper from its sulphide ore.
(ii) Silver and copper articles slowly lose their shiny surfaces when exposed to air. Name the compounds formed on (I) silver articles, and (II) copper articles in the form of coating.
Ans. a. i. I. Ag
II. Al
III. K
IV. Cu
ii. Amphoteric Oxides: Metal oxides that react with both acids, as well as bases to produce salts and water, are called amphoteric oxides. For example ZnO, Al2O3
- Al2O3 + 2NaOH → 3NaAlO2 + H2O
- Al2O3 + 6HCl → 2AlCl3 + 3H2O
iii. Alkalis are bases that are highly soluble in water. An example of an alkali is sodium hydroxide (NaOH).
b. i. Cinnabar (HgS) is an ore of mercury. When it is heated in air, it is first converted into mercuric oxide (HgO). Mercuric oxide is then reduced to mercury on further heating.
Roasting: 2HgS (s) + 3O2 (g) (Heat) → 2HgO (s) + 2SO2 (g)
Reduction: 2HgO (s) (Heat) → 2Hg (l) + O2 (g)
Copper which is found as Cu2S in nature can be obtained from its ore by just heating in air.
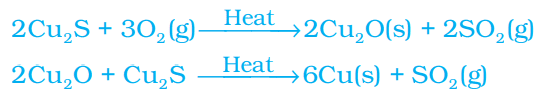
ii. (I) Silver articles form a coating of silver sulphide (Ag2S) when exposed to air.
(II) Copper articles form a coating of copper oxide (CuO) when exposed to air.
35. (a) (i) Identify the parts 'X' and 'Y' in the figure given below:
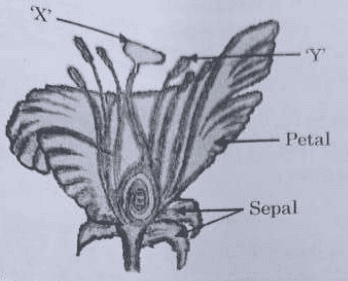
(ii) Name the yellowish coloured structures produced by the part labelled as 'Y'.
(iii) Write the name of the process by which these are transferred to the part labelled as 'X'.
(iv) Explain the process of seed formation in a flowering plant.
OR
(b) (i) Name the type of asexual mode of reproduction shown in the given figure.
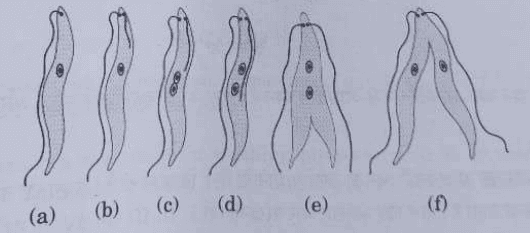
(ii) Identify the unicellular organism in the diagram.
(iii) List any two advantages of asexual reproduction over sexual reproduction.
(iv) Name and explain any one mode of asexual reproduction observed in Hydra.
Ans. a. i. X: 'X' is pointing to the stigma. Y: 'Y' is pointing to the anther.
ii. The yellowish structures produced by the anther are pollen grains.
iii. The process by which pollen grains are transferred from the anther to the stigma is called pollination.
iv. Process of seed formation in a flowering plant:
- The ovule develops a seed coat and turns into a seed.
- The pollen from the stamen is transferred to the stigma.
- The male germ cell and the female germ cells combine to form the zygote.
- The zygote undergoes rapid division to form the embryo inside the ovule.
b. i. Binary fission
ii. Leishmania
iii. Two advantages of asexual reproduction:
- Rapid reproduction
- No need for a mate
iv. Budding in Hydra: A bud develops as an outgrowth or protrusion on the parent Hydra's body. This bud contains cells that are rapidly dividing and differentiating. The bud grows and eventually develops into a new, independent Hydra. Once it's sufficiently developed, it detaches from the parent Hydra and lives on its own.
36. (a) (i) "In refraction of light through a rectangular glass slab, the emergent ray is always parallel to the direction of the incident ray." Why? Explain with the help of a ray diagram.
What happens when a ray of light falls normally on one of the faces of a rectangular glass prism? Draw diagram.
(ii) An object is placed at a distance of 30 cm from the optical centre of a concave lens of focal length 20 cm. Use Lens formula to determine the position of the image formed in this case.
OR
(b) (i) A student wishes to study the image formation by a concave mirror using candle flame as object. State the type of the image formed by the mirror and mention the change in the image formed, if any, that he observes when the candle flame is gradually moved away from the pole of the mirror. Draw a ray diagram to show the image formation when the object distance is nearly equal to the radius of curvature of the mirror.
(ii) A convex mirror used for rear-view on an automobile has a focal length of 3.0 m. If a bus is located at 6.0 m from this mirror, use mirror formula to find the position of the image of the bus as seen in the mirror.
Ans. a. i. Refraction of light through a rectangular glass slab:
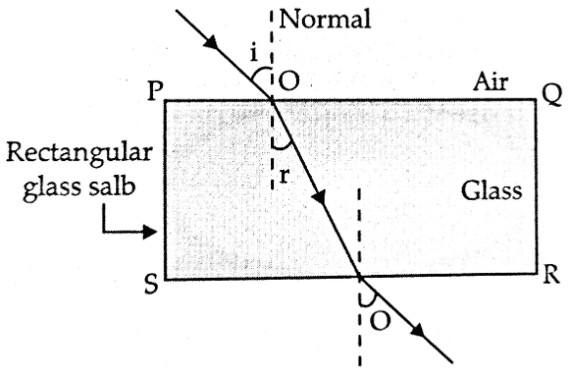
In a rectangular glass slab, the emergent rays are parallel to the incident ray because the extent of bending of the ray of light at the opposite parallel faces of the rectangular glass slab are equal and opposite, so the emergent ray is parallel to the incident ray.
When a ray of light falls normally (at an angle of 0°) on one face of a rectangular glass prism, it passes undeviated through the prism and emerges from the opposite face without any change in direction.
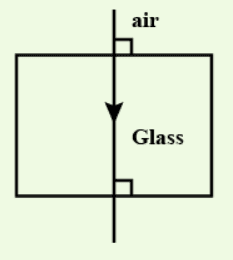
ii. Lens Formula: 1/v - 1/u = 1/f
- v = ?
- u = object distance = -30 cm (negative because it's on the left of the lens)
- f = focal length = -20 cm (negative for a concave lens)
1/v - 1/(-30) = 1/(-20)
1/v + 1/30 = -1/20
1/v = -1/20 - 1/30
1/v = (-3 - 2)/60
1/v = -5/60
v = -12 cm
b. i. When the candle flame is close to the concave mirror, the image formed is virtual, upright, and magnified. As the candle is moved away from the mirror, the image remains virtual and upright but diminishes in size.
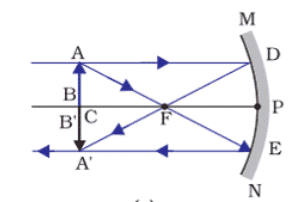
ii. Mirror Formula: 1/v + 1/u = 1/f
- v = image distance (what we want to find)
- u = object distance = -6.0 m (negative because it's on the left of the mirror)
- f = focal length = +3.0 m (positive for a convex mirror)
1/v + 1/(-6) = 1/3
1/v - 1/6 = 1/3
1/v = 1/3 + 1/6
1/v = (2 + 1)/6
1/v = 3/6
1/v = 1/2
v = 2 m
The image of the bus is formed 2 meters behind the mirror. Since v is positive, the image is virtual and upright, as expected for a rear-view mirror.
Section E
The following questions are Source-based/Case-based questions. Read the case carefully and answer the questions that follow.
37. 'A' and 'B' are two salts used for washing purposes. Salt 'A' is used for bathing also. Four test tubes I, II, III and IV as mentioned below are taken.
I. Rain water + solution of salt 'A'
II. Rain water + solution of salt 'B'
III. Tubewell water + solution of salt 'A'
IV. Tubewell water + solution of salt 'B'
The test tubes are shaken one by one almost identically for the same time and the lengths of foam formed in each test tube is noted.
(a) In which one of the four test tubes is the foam formed the minimum?
(b) Differentiate between salt A and salt B.
(c) (i) What are esters? What happens when an ester reacts with an alkali (say sodium hydroxide)? Give chemical equation for the reaction.
OR
(c) (ii) What is the cause of hardness of water? Sometimes it is observed that while bathing foam is formed with difficulty and an insoluble substance is formed. Name this substance and write the cause of its formation.
Ans. a. Since salt 'A' is used for bathing, it is likely to be a soap or a surfactant that forms foam easily. Tubewell water is likely to be hard water, which can reduce foam formation. Therefore, the minimum foam formation is expected in test tube III, which contains tubewell water and salt 'A'.
b. Salt 'A' is used for bathing and forms foam easily, indicating that it is a soap. Salt 'B' is not used for bathing and may not form foam as easily, suggesting that it is a detergent.
| Soap | Detergents |
| 1. Molecules of soap are sodium or potassium salts of long-chain carboxylic acids. | 1. Detergents are generally sodium salts of sulphonic acids or ammonium salts with chloride or bromide ions. |
| 2. Not so effective in hard water. | 2. It is effective even in hard water. |
| 3. It forms scum in hard water. | 3. Does not form scum in hard water. |
| 4. It has poor foaming capacity. | 4. It has a rich foaming capacity. |
| 5. Soaps are biodegradable. | 5. Most of the detergents are non-biodegradable. |
c. i. Esters are organic compounds formed by the reaction between a carboxylic acid and an alcohol, typically in the presence of an acid catalyst. They often have pleasant, fruity smells.
Saponification is the process of converting esters into salts of carboxylic acids and ethanol by treating them with a base.
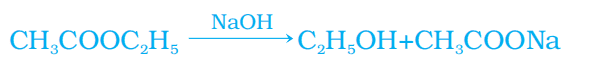
c. ii. Cause of Hardness of Water: Presence of dissolved calcium (Ca²⁺) and magnesium (Mg²⁺) ions in water.
These ions react with soap molecules (salt ‘A’), forming an insoluble precipitate (scum), reducing foam formation.
38. A person while climbing up a rocky hill comes into a panic state and fear. His body starts reacting in a "flight-or-flight" condition to adjust to the dangerous and stressful situation.
Based on the above facts, answer the questions that follow.
(a) (i) Name the hormone secreted in the blood of the person in this situation.
OR
(a) (ii) Name the source gland of the hormone secreted in this condition.
(b) State any two responses in the body of the person as a result of the secretion of this hormone.
(c) How does the action of the chemical signal in terms of hormones differ from the electrical impulses via nerve cells?
Ans. a. i. The hormone secreted in response to stress is adrenaline.
a. ii. The source gland of adrenaline is the adrenal gland.
b. Two responses in the body due to adrenaline secretion are:
- Increased heart rate and blood pressure to prepare the body for "fight or flight".
- Increased glucose release from stored energy sources to provide a quick energy boost.
c.
| Nervous System | Hormonal System |
| 1. Fast speed | 1. Slow speed |
| 2. Electrical impulses travel through nerve fibre | 2. Chemical messengers (hormones) travel through blood. |
| 3. Short-lived effect | 3. Long-lasting effect |
| 4. Cannot reach all cells in the body. | 4. Can reach all cells in the body. |
39. As shown in the diagram, an electric circuit consisting of an ammeter, a voltmeter, 4 cells of 1.5 V each, a plug key with a gap XY was set up. Voltmeter and ammeter readings were recorded in the observation table for four arrangements as given below :
- Arrangement No. 1 - only resistor R₁ in gap XY
- Arrangement No. 2 - only resistor R₂ in gap XY
- Arrangement No. 3 - Resistors R₁ and R₂ in series in gap XY
- Arrangement No. 4 - Resistors R₁ and R₂ in parallel in gap XY
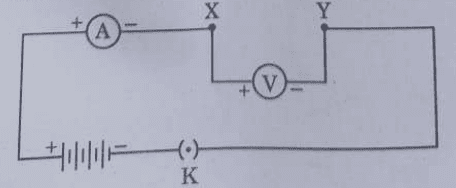
Based on the observations, four V-I graphs A, B, C and D as shown in figure were drawn. Study these graphs.
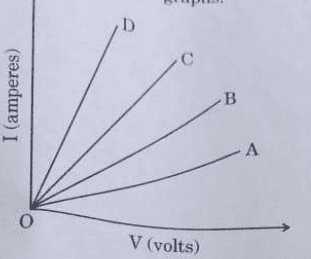
(a) Which one of the graphs represents the series combination of R₁ and R₂?
(b) Which one of these graphs represents the parallel combination of R₁ and R₂?
(c) (i) Show an arrangement of three resistors, each of resistance 10 Ω, so that the combination has a resistance of 15 Ω. Give justification for your answer.
OR
(c) (ii) A battery of 6 V is connected with a series combination of five resistors of 0.1 Ω, 0.2 Ω, 0.3 Ω, 0.4 Ω and 0.5 Ω. How much current would flow through the 0.3 Ω resistor? Justify your answer.
Ans. a. Graph A
b. Graph D
c. i. We can combine two resistors in parallel and then connect this combination in series with the third resistor.
For the parallel combination, 1/R_parallel = 1/10 + 1/10 = 2/10.
R_parallel = 10/2 = 5 Ω.
Then, for the series combination,
R_total = R_parallel + 10 = 5 + 10 = 15 Ω.
(c) (ii) Current Flow through the 0.3Ω Resistor in a Series Circuit
Given:
- Battery Voltage: 6V
- Resistors: 0.1Ω, 0.2Ω, 0.3Ω, 0.4Ω, 0.5Ω (all in series)
Finding Total Resistance
Rtotal=0.1+0.2+0.3+0.4+0.5=1.5Ω
V=IR
⇒I=V/Rtotal
⇒I = 6/1.5 = 60/15 =4A
In a series circuit, current remains the same across all resistors.
So, the current through the 0.3Ω resistor is also 4A.
CBSE Class 10 Science Answer Key PDF – Download Now!
Students can download the CBSE Class 10 Science Answer Key 2025 PDF from the link below:
🔽 Download PDF (Link will be updated soon)
CBSE Class 10 Science Exam 2025: Student Reactions & Difficulty Level
Based on student feedback, the CBSE Class 10 Science 2025 exam was Easy to Moderate. Many students found Physics numericals tricky, while Chemistry and Biology were balanced.
✍️ Student Feedback:
- "Physics numericals were tough but manageable."
- "Chemistry had straightforward reaction-based questions."
- "Biology diagrams were simple and easy to label."
CBSE Class 10 Science 2025 Exam: Expected Cut-Off & Analysis
- Easy Paper: Expected average score – 75-80 marks
- Moderate Paper: Expected average score – 65-75 marks
- Difficult Paper: Expected average score – 50-65 marks
The official answer key and marking scheme will be updated soon. Stay tuned!
FAQs on CBSE Class 10 Science Answer Key 2025
❓ When will CBSE release the official answer key?
✔️ CBSE does not officially release answer keys for subjective exams, but unofficial answer keys are available on our website.
❓ Where can I download the answer key for the CBSE Class 10 Science 2025 exam?
✔️ You can download the CBSE Class 10 Science Answer Key 2025 PDF from CBSEGuidanceWeb.com.
❓ How can I check my expected marks?
✔️ Compare your answers with our detailed CBSE Class 10 Science Answer Key 2025 and follow the CBSE marking scheme.
Stay Connected for More Updates!
📢 Follow Us:
✔️ YouTube: CBSE Guidance
✔️ Website: www.cbseguidanceweb.com
✔️ WhatsApp: Join our WhatsApp channel for instant updates!
💬 Comment Below: Share your exam experience and discuss answers with fellow students!
🚀 Stay tuned for more CBSE Class 10 exam solutions, study materials, and important updates.
Sir please Reply me I HAVE Drawn WRONG DIAGRAM IN Q31 THEN HOW MANY MARKS WOULD BE DEDUCTED
02 marks for diagram of rainbow formation.
If in case study questions no 39 graph a,,b
A) a. And b
B) c. And d
I have written then how much marks will deduct
If in case study questions no 39 graph a,,b
A) a. And b
B) c. And d
I have written then how much marks will deduct
Please give answer
no marks deducted
Sir plz reply.
In set 3 Q 21 I had accidentally mentioned the reaction as exothermic even though the I Wrote the reason for endothermic ( ans was endothermic)
How many marks would be deducted?
Give me the question please.
name the compound used in black and white photography also state whether the reaction is endothermic or exothermic with reason
It was a 2 marks question
You will get 01 marks, if “endothermic” word is not there in your answer.
Endothermic because energy or heat is absorb in the form of sunlight
Sodium chloride and sodium bromide
Silver Chloride is used
It’s an photolytic decomposition reaction and endothermic reaction.
AgCl2 ——–> Ag + Cl2
Set -3
Ques 1
Sir option c bhi to thik hai kyonki so isomers and related pucha hai na ki same.
Haa Option C he sahi hai.
Sir q-19 Ka answer b hoga magnetic field wale ka
I wrote the correct reason but
Still?
Sir question number 32 me diagram wale part ka kitna weightage hoga,mera baaki dono parts sahi hai but diagram galat hai
01 Mark
Sir I have written wrong spelling of adrenaline and brine, just minor mistake. Will I get marks?
Depends on the examiner. If he wishes, he can give marks.
If in case study questions no 39 graph a,,b
A) a. And b
B) c. And d
I have written then how much marks will deduct
Sir can u pls tell the answer of this assertion reasoning question:
Assertion (A) : In large animals, oxygen can reach different parts of the
animal’s body easily.
Reason (R): Respiratory pigments take up oxygen from the air and
carry it to body tissues.
A is false, R is true
Sir, is the given answer key correct?
There are many answers which are wrong.
Tell me the question numbers, I will recheck them.
Sir set 1 m ques no. 25 ka answer galat h it should be hypermetropia
Thanks for telling. Corrected.
sir physics case study me qsn me toh specify kara tha ki v-i graph tha but graph given i-v tha , tho mene v-i graph ki according answer kiya, qsn me printing mistake tha na tho usme mark kese di jayaga?
Sir mera set 2 tha and mera 4 marks ka case based question choot gya 😭 sir sab aata tha fir bhi ye hogya
Paper mei question line wise attempt krne hote hai yaa chem,phy,bio teeno ko seperate seperate kyuki hamare school mei toh seperate karvate the
Line wise..as provided on question paper…not separately..
In question no 37 sir I have not differentiate but wrote about soap and detergent how many mark will be deducted??
01 Mark only
Sir I think 4 MCQ is incot
Sir option c bhi to thik hai kyonki so isomers and related pucha hai na ki same.
Sir mene Ray diagram wale question mai convex ki jaagh concave draw kiya , lekin mene type of image, size and backing sab likha hai , mere kitne marks kutenge , 3 marks k question hai
02 Marks
Sir, Set 2 answer key is not provided yet….only set 1 answer is there
Please provide set 2 answer key too…
Set 3 is very hard
Set 3 was soo easy. All are direct questions but set 2 was difficult and tooo lengthy. Why cbse shows this type of discrimination in sets. This is too bad.
Check set 31/3/3, literally the hardest
Sir from where to see set 3 answers
Sir please pass kardena set 1 bohot hard tha as compare to all
Sir 50 up number de Dena sir please hard paper tha … 🥺
Sir ,in the physics case study qsn it was mentioned that the given graph was
V-I , but they provided with an I-V graph
I followed the qsn and answered according to the V-I graph, how will be the marks awarded?
You should have answered the question by looking at the graph (IV graph). So I think you have made mistake in part (a) and (b). So 02 marks will be cut.
Sir in set 2 there was a question that name the information source for making proteins in our body I have written dna is it correct?
Sir in my set there was a question where we had to tell a reason for a given reaction, I wrote the answer which was oxidation but I also wrote endothermic along side that and apparently it was exothermic. Will i get my marks deducted for that? And in the ray diagram for the correction of myopia, I didn’t draw the dotted lines, will I get my marks deducted? Overall the diagram was correct
If we wrote that alkali are bases that get completely dissolved in water to produce oh- ions. Ex- Naoh