Hey Class 10 science warriors! 👋
Feeling overwhelmed by Carbon and Its Compounds (Chapter 4) for your upcoming CBSE board exams? 😩 Don't worry, we've got your back! 💪
This blog post is your one-stop shop for mastering this crucial chapter. 🏆 We've compiled the most effective Class 10 Carbon and Its Compounds notes in a downloadable PDF format, just for you! 🎁
But wait, there's more! 🤩 These aren't just any notes. We've carefully crafted them to be clear, concise, and packed with real-world examples that will make even the trickiest concepts stick. 🧠
Ready to ace your Carbon and Its Compounds test and impress your teachers? 😎 Let's dive in!
| Subject | Science (Chemistry) |
| Class | 10 |
| Board | CBSE & State Boards |
| Chapter No. | 4 |
| Chapter Name | Carbon and its Compounds |
| Type | Notes |
| Session | 2024-25 |
"The way to get started is to quit talking and begin doing."
- Walt Disney
Carbon and Its Compounds Class 10 Notes
Table of Contents
Bonding in Carbon - The Covalent Bond
| Element | Carbon |
| Atomic Number | 6 |
| Electronic Configuration | 2, 4 |
| Valence Electrons | 4 (Carbon is tetravalent because it has 4 valence electrons) |
| Valency | 4 |
Carbon neither gains nor loses 4 electrons to attain the noble gas electronic configuration because:
- It could gain four electrons forming C4– anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons.
- It could lose four electrons forming C4+ cation. But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
Carbon overcomes this problem by sharing its valence electrons with other atoms of carbon or with atoms of other elements.
The shared electrons ‘belong’ to the outermost shells of both the atoms and lead to both atoms attaining the noble gas configuration.
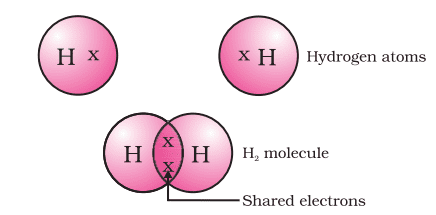
Formation of Hydrogen molecule (H2)
Formation of Oxygen Molecule (O2)
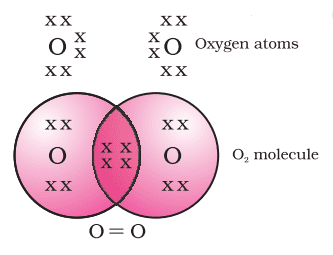
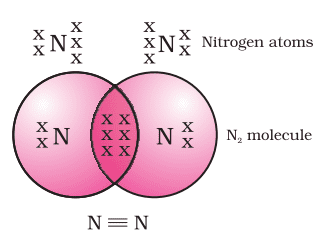
Formation of Nitrogen Molecule (N3)
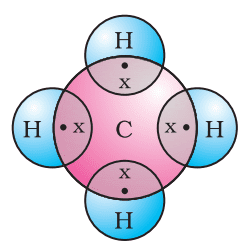
Formation of Methane (CH4)
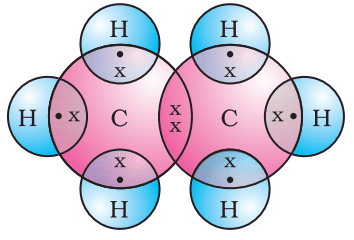
Formation of Ethane (C2H6)
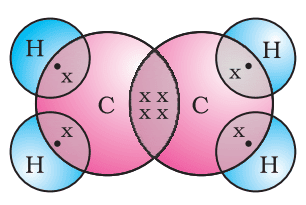
Formation of Ethene (C2H4)
Covalent Bond
Such bonds which are formed by the sharing of an electron pair between two atoms are known as covalent bonds.
Features of Covalent Compounds
- Covalent compounds do not form ions, hence they are bad conductors of electricity.
- The forces of attraction between the molecules are not very strong. Hence, covalent compounds have low melting and boiling points.
Versatile Nature of Carbon
The two properties of carbon that led to the huge number of carbon compounds are:
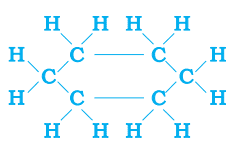
- Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation. (Carbon exhibits catenation much more than silicon or any other element due to its smaller size which makes the C-C bonds strong while the Si-Si bonds are comparatively weaker due to their larger size.)
- Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element (Tetravalency).
Saturated and Unsaturated Carbon Compounds
| Saturated Hydrocarbons | Unsaturated Hydrocarbons |
| 1. Compounds of carbon having only a single bond between their carbon atoms are called saturated hydrocarbons. | 1. Compounds of carbon having double or triple bonds between their carbon atoms are called unsaturated hydrocarbons. |
| 2. Less reactive than unsaturated hydrocarbons. | 2. More reactive than saturated hydrocarbons. |
| 3. General formula is CnH2n+2. | 3. General formula is CnH2n (alkenes) or CnH2n-2 (alkynes). |
| 4. It burns with a clean flame. | 4. It burns with a yellow sooty flame. |
| 5. Substitution reaction is the characteristic property of these hydrocarbons. | 5. Addition reaction is the characteristic property of these hydrocarbons. |
Hydrocarbons: Carbon compounds that contain only carbon and hydrogen are called hydrocarbons.
Alkanes: The saturated hydrocarbons are called alkanes.
Alkenes: The unsaturated hydrocarbons which contain one or more double bonds are called alkenes.
Alkynes: The unsaturated hydrocarbons which contain one or more triple bonds are called alkynes.
Structural isomers: They have the same molecular formula but they differ in their structures.
Structural isomers of C4H6 are
- HC ≡ C - CH2 - CH3
- H3C - C ≡ C - CH3
- H2C = C = CH - CH3
- H2C =CH - CH =CH2
Saturated Ring: Example: cyclohexane (C6H12)
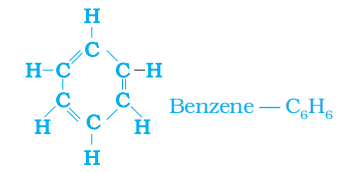
Unsaturated Ring: Example: benzene (C6H6)
Will you be my Friend?
Homologous Series: A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series.
Example: CH3OH, C2H5OH, C3H7OH, C4H9OH, ….
Characteristics of homologous series:
- It has a general formula in terms of the number of carbon atoms.
- It has the same functional group.
- The members of a homologous series i.e., homologues, have similar chemical properties.
- The members of a homologous series i.e., homologues, show a gradation in physical properties with an increase in molecular mass.
- Formulae of two successive homologues differ by -CH2- unit.
- The difference in molecular mass between two successive homologues is 14 u.
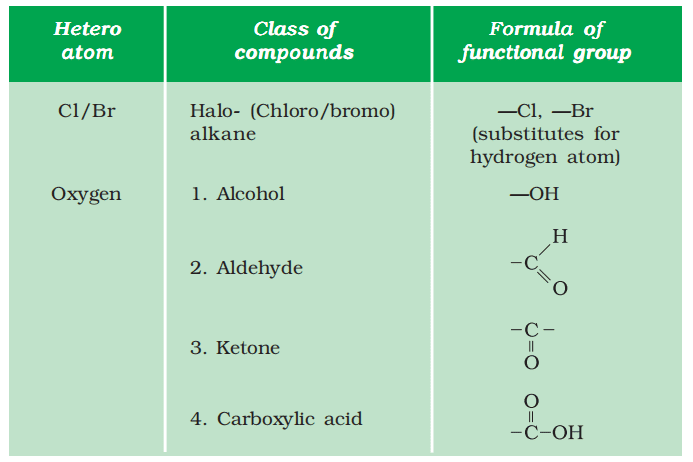
Nomenclature of Carbon Compounds
For nomenclature, watch the below video from 2:15:41
Chemical Properties of Carbon Compounds
Combustion
Carbon burns in oxygen to give carbon dioxide along with the release of heat and light.
- C + O2 → CO2 + heat and light
- CH4 + O2 → CO2 + H2O + heat and light
- CH3CH2OH (ethanol) + O2 → CO2 + H2O + heat and light
Oxidation
Carbon compounds can be easily oxidised on combustion.
Reactions in which alcohols are converted to carboxylic acids.

Alkaline potassium permanganate or acidified potassium dichromate are oxidising alcohols to acids, that is, adding oxygen to the starting material. Hence they are known as oxidising agents.
Addition Reaction
Hydrogenation: The addition of hydrogen to an unsaturated hydrocarbon to get a saturated hydrocarbon in presence of nickel or palladium as catalyst is called hydrogenation.
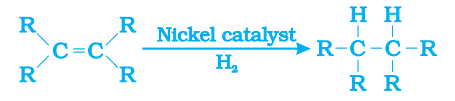
This reaction is commonly used in the hydrogenation of vegetable oils using a nickel catalyst.
Vegetable oils generally have long unsaturated carbon chains while animal fats have saturated carbon chains.
Vegetable oils are ‘healthy’. Animal fats generally contain saturated fatty acids which are said to be harmful for health. Oils containing unsaturated fatty acids should be chosen for cooking.
Substitution Reaction
In the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction. Chlorine can replace the hydrogen atoms one by one.
CH4 + Cl2 → CH3Cl + HCl (in the presence of sunlight)
Some Important Carbon Compounds - Ethanol and Ethanoic Acid
Properties of Ethanol
- Ethanol is a liquid at room temperature.
- Ethanol is commonly called alcohol and is the active ingredient of all alcoholic drinks.
- Ethanol is used to make cough syrups, tincture of iodine, etc.
- It is also used as a fuel as an additive to petroleum.
- Ethanol is soluble in water in all proportions.
Reactions of Ethanol
1. Reaction with sodium
2Na + 2CH3CH2OH → 2CH3CH2O–Na+ (sodium ethoxide) + H2
Alcohols react with sodium leading to the evolution of hydrogen. With ethanol, the other product is sodium ethoxide.
2. Reaction to give unsaturated hydrocarbon
Heating ethanol at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethene.

The concentrated sulphuric acid can be regarded as a dehydrating agent which removes water from ethanol.
Properties of Ethanoic Acid
- Ethanoic acid is commonly called acetic acid and belongs to a group of acids called carboxylic acids.
- 5-8% solution of acetic acid in water is called vinegar and is used widely as a preservative in pickles.
- The melting point of pure ethanoic acid is 290 K and hence it often freezes during winter in cold climates. This gave rise to its name glacial acetic acid.
- Carboxylic acids are weak acids.
Reactions of ethanoic acid
1. Esterification reaction: Esters are most commonly formed by reaction of an acid and an alcohol. Ethanoic acid reacts with absolute ethanol in the presence of an acid catalyst to give an ester.
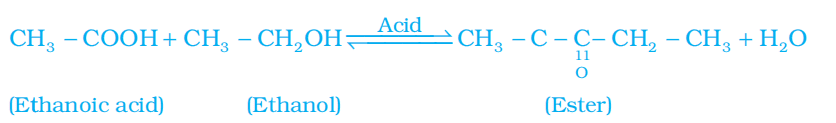
- Esters are sweet-smelling substances. These are used in making perfumes and as flavouring agents.
- Saponification is the process of converting esters into salts of carboxylic acids and ethanol by treating them with a base.
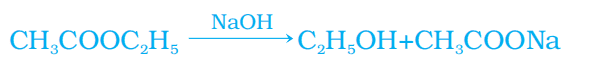
2. Reaction with a base
Ethanoic acid reacts with a base to give salt and water.
NaOH + CH3COOH → CH3COONa (sodium ethanoate/sodium acetate) + H2O
3. Reaction with carbonates and hydrogen carbonates: Ethanoic acid reacts with carbonates and hydrogen carbonates to give rise to salt, carbon dioxide, and water.
- 2CH3COOH + Na2CO3 → 2CH3COONa (sodium acetate) + H2O + CO2
- CH3COOH + NaHCO3 → CH3COONa (sodium acetate) + H2O + CO2
| Ethanol | Ethanoic Acid |
| (Difference in Physical properties) | |
| 1. It exists only in liquid form. | 1. It can exist both in liquid and solid form. |
| 2. Functional group is alcohol. | 2. Functional group is carboxylic acid. |
| 3. It has a specific smell but not like vinegar. | 3. It smells like vinegar. |
| (Difference in Chemical properties) | |
| 4. Does not react with sodium bicarbonate. | 4. Reacts with sodium bicarbonate to form salt, carbon dioxide, and water. |
| 5. No effect on litmus paper. | 5. It turns blue litmus paper red. |
Soaps and Detergents
Cleansing action of soaps
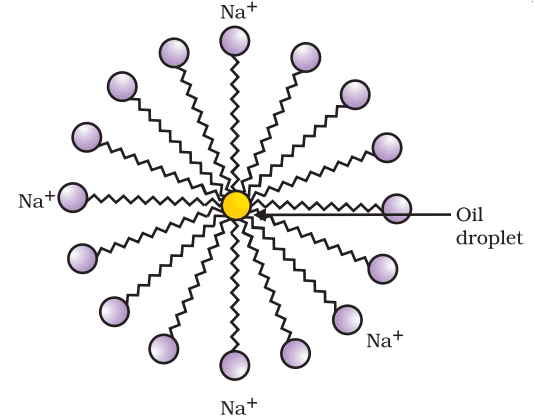
Most dirt is oily in nature and oil does not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic end of soap interacts with the water while the carbon chain interacts with oil. The soap molecules, thus form structures called micelles where one end of the molecules is towards the oil droplet while the ionic end faces outside. This forms an emulsion in water. The soap micelle thus helps in pulling out the dirt in water and we can wash our clothes clean.
| Soap | Detergents |
| 1. Molecules of soap are sodium or potassium salts of long-chain carboxylic acids. | 1. Detergents are generally sodium salts of sulphonic acids or ammonium salts with chloride or bromide ions. |
| 2. Not so effective in hard water. | 2. It is effective even in hard water. |
| 3. It forms scum in hard water. | 3. Does not form scum in hard water. |
| 4. It has poor foaming capacity. | 4. It has a rich foaming capacity. |
| 5. Soaps are biodegradable. | 5. Most of the detergents are non-biodegradable. |
- The soluble salts of calcium and magnesium make the water hard.
- Scum is formed by the reaction of calcium or magnesium ions with soap molecules. These ions react with soap molecules forming calcium or magnesium salts of long-chain carboxylic acids which are insoluble and precipitate out.
- Detergents are generally sodium salts of sulphonic acids or ammonium salts with chlorides or bromides ions, etc.
- The charged ends of these compounds do not form insoluble precipitates with the calcium and magnesium ions in hard water. Thus, they remain effective in hard water.
Chapter 4 Carbon and its Compounds Class 10 NCERT Underlined PDF
| Must Read: Carbon and its Compounds Class 10 Important Questions with Answers to get an idea of the different types of questions asked from this chapter. |
| You Might Also Like: CBSE Class 10 Notes CBSE Class 10 Important Questions and Answers |
Hope you liked these notes on Class 10 Science Chapter 4 Carbon and its Compounds. Please share this with your friends and do comment if you have any doubts/suggestions to share.
No option for downloading the pdf …..
How to Download PDF from CBSE Guidance Website
https://youtu.be/zjocgf0LzUM
Very nice notes detailed information and explanation because of this notes I achieved 98 marks in science class 10 board exam
notes are so beautiful so elegant just looking like a wow he prabhu he hariram krishna jagganatham he kya hua aeeeee iss notes ke aage koi bol sakta he kya .com moye moye
Is it for 2024-25 ??